How Do the Molecules of a Gas Behave
2 How do water molecules behave. Liquid In the liquid state particles can move - the movement temperature dependent tends to be less frantic than in the gaseous state.
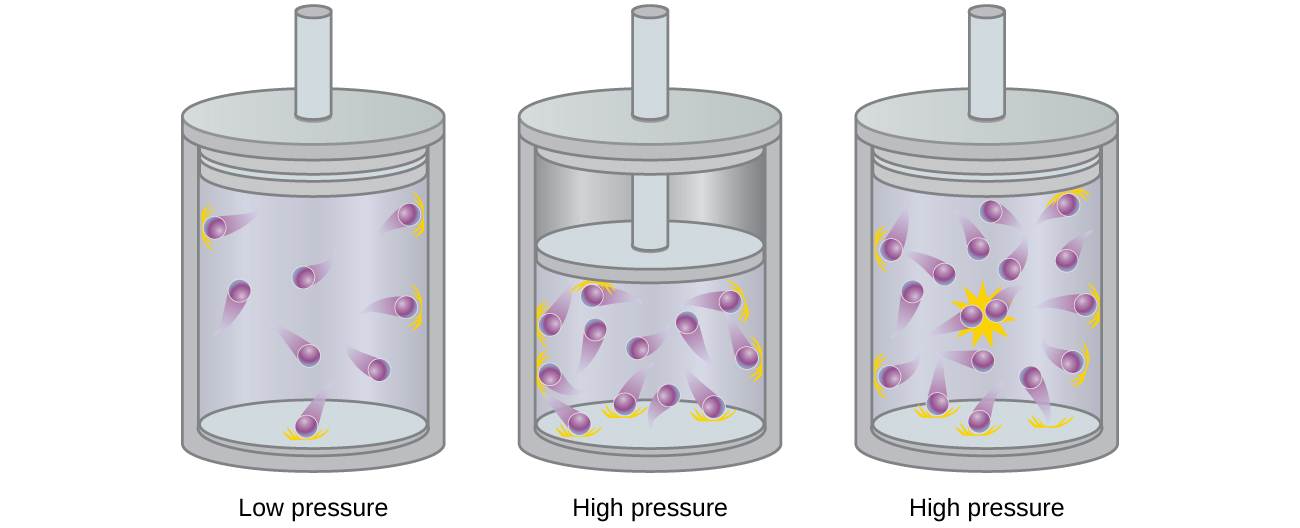
9 6 Non Ideal Gas Behavior Chemistry
For example the volume of a gas can change due to pressure but the volume of a solid or liquid generally cannot.
. 8 What happens when water changes to ice. What element is a gas and can behave as an alkali metal. 10 What is true about.
8 How will you describe the particles in solid the particles in solid are. Solve any question of Kinetic Theory with-. The distribution of molecules in a gas is very different from the distribution of molecules in liquids and solids.
The molecules behave as rigid spheres. Solid vibrate jiggle but generally do not move from place to place. When samples of gases are placed in.
9 What is true about atoms and molecules quizlet. Water can exist as a solid ice liquid water liquid or gas water vapor. 13 What are the three factors that determine the state.
The basic molecular formula for the water molecule is the same in each H 2O. The reason is that ordinarily only statistical averages are observed in the study of the behaviour and properties of gases and statistical methods are quite accurate when large numbers are involved. The enormous number of molecules in even a small volume of a dilute gas produces not complication as might be expected but rather simplification.
Gas vibrate and move freely at high speeds. 8 Which statement about atoms and molecules is correct quizlet. Pressure is due to collisions between the molecules and the walls of the container.
Up to 24 cash back How Do Gas Molecules Behave. There are five properties and five gas laws that govern the behaviour of gas molecules. If you put a thermometer into a pot of melting ice when will the temperature rise past zero.
The behaviour of gas molecules is dependent on the properties and laws obeyed by the molecules of the gas. How do molecules of a gas behave. 4 How do atoms behave.
But as the temperature of the system changes the hydrogen bonds between water molecules change drastically. 7 Why does water behave when frozen. All collisions both between the molecules themselves and between the molecules and the walls of the container are perfectly elastic.
Its either they vibrate in place or they clump together or they bounce around randomly. 3 How are water molecules behaving when they are in ice. 11 What are the two factors that affect the state of matter.
4 Are molecules closer in ice or water. 5 What makes matter do what it does Why do atoms behave the way that they do. Gas In the gaseous state particles move freely at a frantic pace.
Gas molecules are free to move to fill the entire space of their container. Similarly you may ask how do molecules move in a gas. These Molecules collide with one another and with anything else they come in contact With causing pressure.
Gas can be compressed much more easily than a liquid or solid. Gas expands the quickest because gas molecules are farther apart than molecules of other substances. 6 How plasma is different from gas.
4 How the molecules in a solid liquid and gas compare to each other. Click to see full answer. Gas molecules are not held rigidly in place as would be a solid or a lattice of ionic bonds instead gas molecules are constantly in motion and each and every different gas exerts a specific pressure.
They bounce around randomly. 10 How do molecules behave in a gas. What do water vapor liquid water and ice have in common.
5 How are the particles of matter affect its states as to solid liquid and gas. Gas molecules also move faster. They exist in a regular arrangement - there is no regular arrangement in the other states.
9 How does the movement of particles change from solid to. 7 How do molecules of a substance behave during a phase change. The particles are still relatively close together.
7 Is the Sun Fire or plasma. Liquid vibrate move about and slide past each other. 9 Why is the arrangement of water.
6 How are molecules in each phase of matter. Whats the easiest way to add energy to matter. 5 How do water molecules behave in different temperatures.
The same chemical properties. Gases behave differently than solids or liquids do. 12 Why are changes in matter important.
The molecules are in constant random motion and frequently collide with each other and with the walls of any container. 6 How does water behave when frozen. 9 Why do molecules behave differently at each phase.
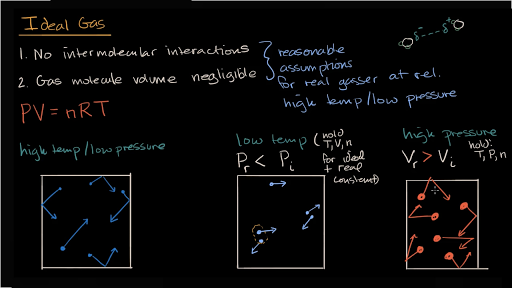
Introduction To Real Gases Video Khan Academy
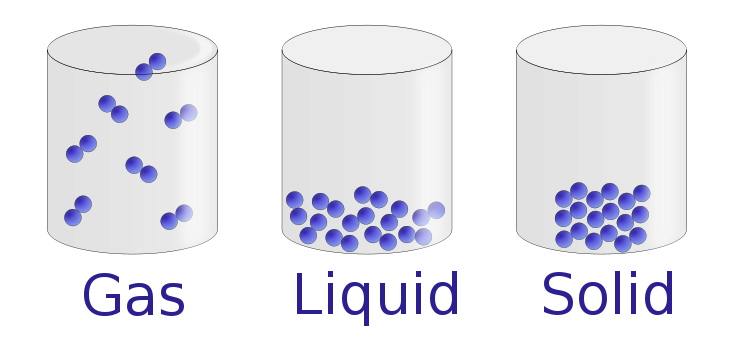
12 1 A Molecular Comparison Of Gases Liquids And Solids Chemistry Libretexts
How Are Particles Arranged In The Three States Of Matter Quora
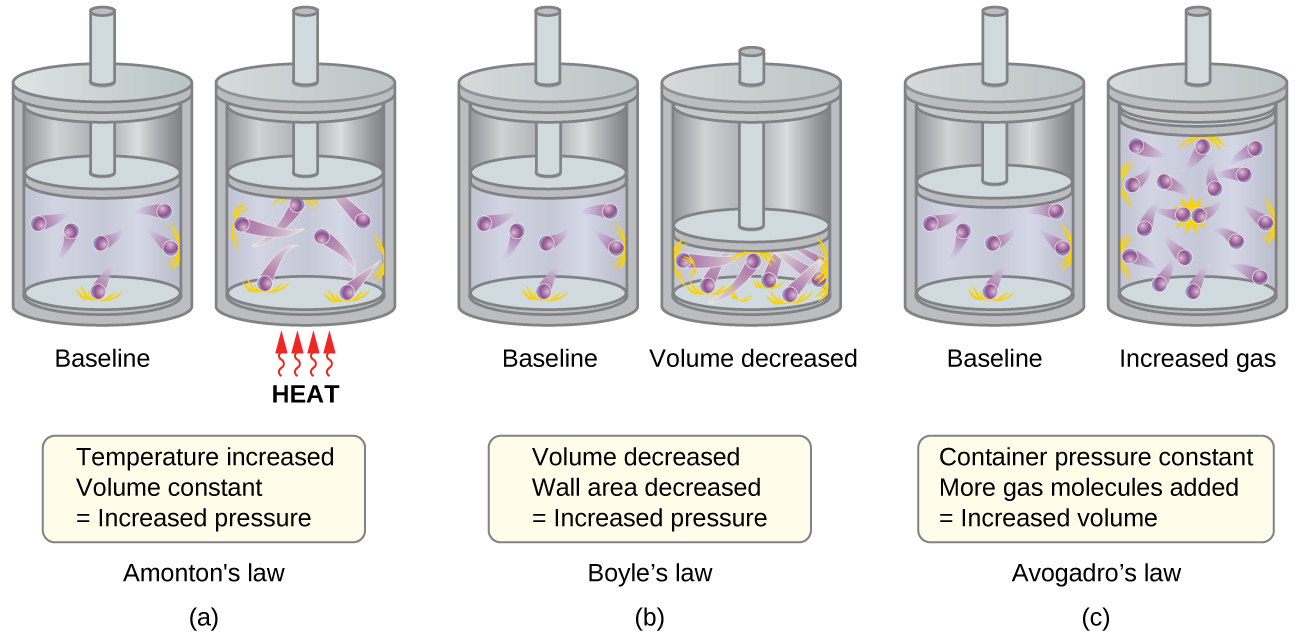
9 5 The Kinetic Molecular Theory Chemistry

The Behavior Of Gases Chemistry For Non Majors
How Are Molecules In A Solid Different From Molecules In A Liquid Quora

Gas State Of Matter Britannica

Kinetic Molecular Theory Of Gases Practice Problems Youtube

Kinetic Theory Of Gases Explanation Assumptions Postulates Formula
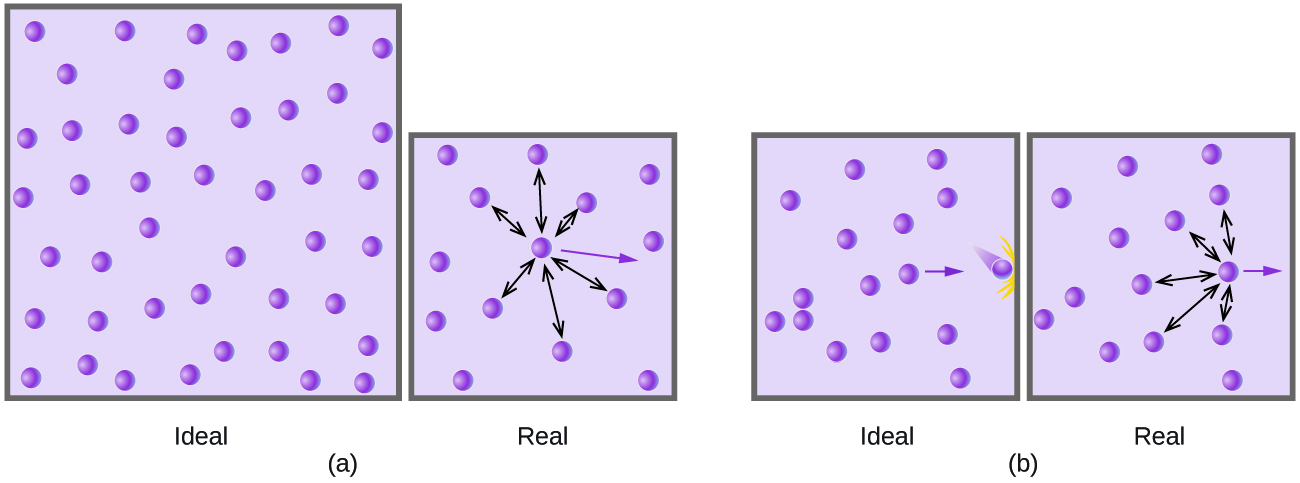
9 6 Non Ideal Gas Behavior Chemistry
Kinetic Molecular Theory Of Gases Let S Talk Science
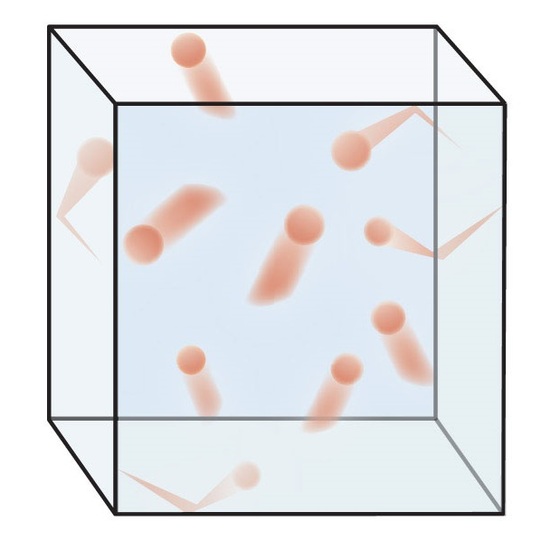
5 5 The Kinetic Molecular Theory A Model For Gas Behavior Chemistry Libretexts
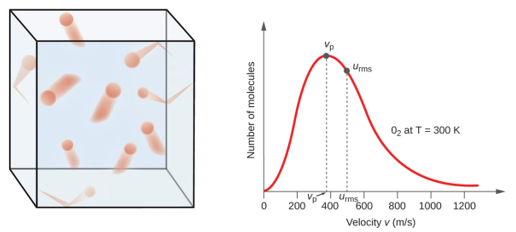
10 5 Kinetic Molecular Theory Of Gases Chemistry Libretexts
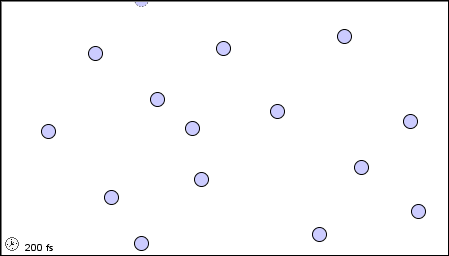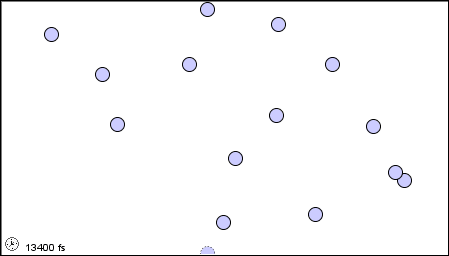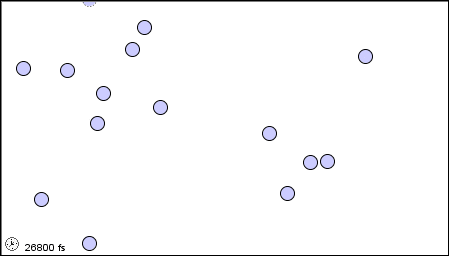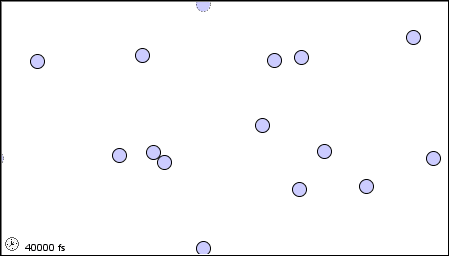
11 1 A Molecular Comparison Of Gases Liquids And Solids Chemistry Libretexts

Comments
Post a Comment